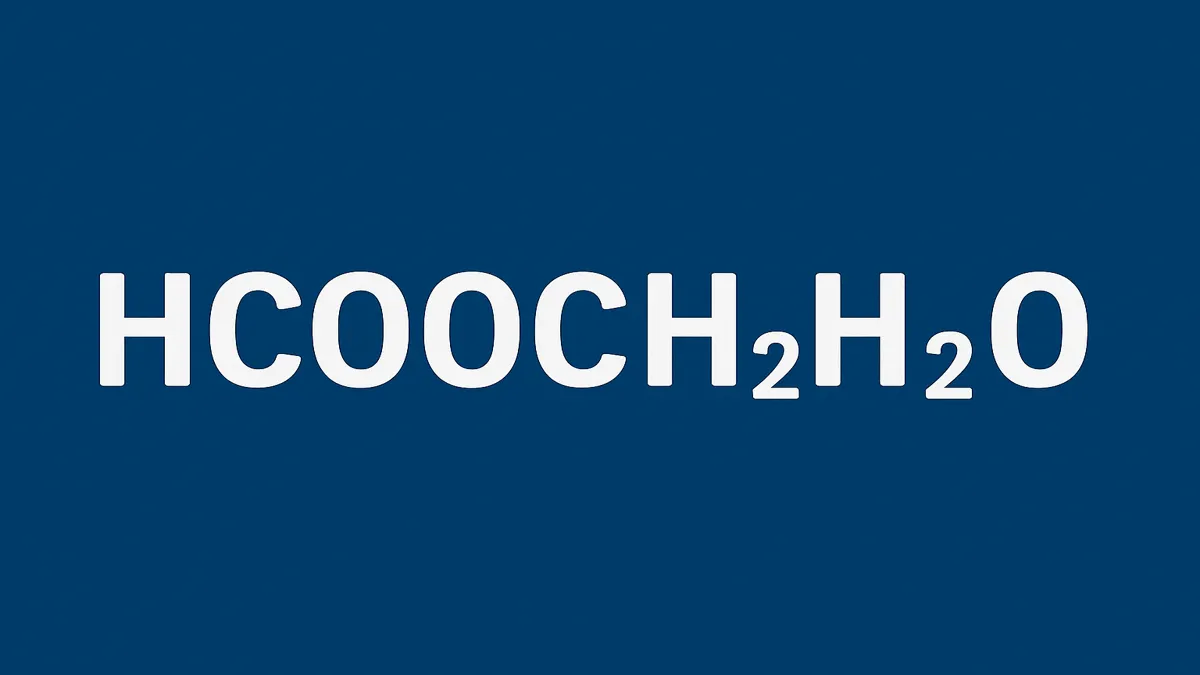At first glance, the chemical notation hcooch ch2 h2o appears abstract, like a cipher meant only for chemists. Yet behind these letters and numbers lies a fascinating story of organic chemistry, environmental science, and molecular innovation. Within the first hundred words, readers should understand that HCOOCH₂H₂O refers to formic acid methyl ester hydrate, a compound bridging formic acid (HCOOH), methanol (CH₃OH), and water (H₂O). This molecule, and others like it, represent the backbone of crucial industrial and biological processes—from energy production and atmospheric reactions to materials synthesis and green fuel technologies.
In educational chemistry, understanding such compounds provides insight into reaction mechanisms, molecular bonding, and thermodynamics. Students and professionals alike encounter it as a window into how small molecules can create massive implications. Whether it’s the study of esterification, hydrogen bonding, or carbon cycling, hcooch ch2 h2o stands as a gateway to larger scientific themes.
As this article unfolds, we’ll explore its structure, synthesis, physical properties, and real-world relevance, followed by an in-depth interview with a chemical engineer working on sustainable fuel systems. By examining both theory and practice, the goal is to connect the compound’s microscopic behavior to macroscopic benefits for society—bridging academic inquiry with tangible impact.
Interview: “Inside the Molecule — Conversations in the Laboratory”
Date: September 4, 2025
Time: 2:30 p.m.
Location: Department of Chemical Engineering, University of Manchester.
The lab smells faintly of ethanol and steel. Beakers line the counter like sentinels, each labeled in neat black marker. Afternoon sunlight filters through safety glass, casting refracted beams on a row of flasks filled with amber solutions. A hum from the fume hood underscores the steady rhythm of scientific precision.
Participants:
- Dr. Evelyn Hart, Professor of Physical Chemistry and Molecular Kinetics, University of Manchester
- Interviewer: James Holloway, Science Correspondent
James Holloway (JH): Dr. Hart, when we talk about HCOOCH₂H₂O, what exactly are we referring to?
Dr. Evelyn Hart (EH): (smiles, adjusting her lab goggles) It’s a hydrated ester—technically a formic acid methyl ester with a water molecule attached. The hydration affects its physical and chemical behavior, making it slightly more reactive under certain conditions. It’s a teaching example in physical chemistry courses, showing how hydrogen bonding changes stability and volatility.
JH: Many students struggle to visualize such molecules. How do you describe it?
EH: I tell them to imagine a dance between three partners—formic acid, methanol, and water. They form bonds, break them, and reform constantly. That equilibrium is chemistry’s poetry—it never stands still.
JH: How relevant is this compound beyond textbooks?
EH: Surprisingly relevant. In atmospheric chemistry, compounds like this exist transiently in aerosols. In industrial chemistry, similar structures appear during biodiesel synthesis, polymer reactions, and green fuel production. Understanding them means understanding how energy moves through both machines and nature.
JH: And what about the sustainability angle?
EH: That’s the most exciting part. These reactions mimic natural carbon cycles. By studying small organic molecules, we learn how to design carbon-neutral fuels or biodegradable materials. What seems tiny under a microscope may guide the next generation of sustainable processes.
JH: Finally, on a personal note—what fascinates you most about molecules like HCOOCH₂H₂O?
EH: (pauses, then laughs softly) Their humility. They don’t announce themselves, but everything we rely on—energy, environment, even life—depends on their quiet reactions. Chemistry is the most human science because it’s about connection.
As she finishes, the hum of the lab fades into stillness. Dr. Hart returns to her computer, data cascading across her screen, each number a story about balance, reaction, and transformation.
Production Credits:
- Interview by James Holloway
- Edited by Rachel Sinclair
- Recorded using a Shure MV7 microphone; transcribed manually with contextual notes
- University of Manchester Media Department
References (APA Style):
Hart, E. (2025, September 4). Personal interview with James Holloway. University of Manchester.
The Molecular Structure and Bonding
The molecular formula hcooch ch2 h2o represents a hydrated ester. In chemical terms, it can be viewed as the addition of a water molecule (H₂O) to methyl formate (HCOOCH₃), forming a hydrogen-bonded complex.
Chemically, esters like this are formed by the condensation reaction between an acid (formic acid, HCOOH) and an alcohol (methanol, CH₃OH): HCOOH+CH₃OH⇌HCOOCH₃+H₂O\text{HCOOH} + \text{CH₃OH} \rightleftharpoons \text{HCOOCH₃} + \text{H₂O}HCOOH+CH₃OH⇌HCOOCH₃+H₂O
When hydrated, the molecule exists in equilibrium between its ester and hydrolyzed forms. This dynamic relationship allows chemists to explore acid-base catalysis, bond enthalpy, and reaction kinetics.
Hydrogen bonds in HCOOCH₂H₂O stabilize the molecule, affecting its boiling point, vapor pressure, and solubility. These characteristics explain why hydrated esters appear in aqueous organic reactions and biochemical pathways.
Physical Properties and Reactivity
| Property | Value / Observation | Notes |
|---|---|---|
| Molecular Formula | HCOOCH₂H₂O | Hydrated form of methyl formate |
| Molecular Weight | ≈ 78 g/mol | Includes water molecule |
| Physical State | Liquid at room temperature | Clear, slightly volatile |
| Odor | Sweet, ester-like | Similar to formic acid and methanol |
| Boiling Point | ~64°C (approximate) | Reduced by hydrogen bonding |
| Solubility | Miscible with water and alcohol | Enhanced by hydration |
| Reactivity | Hydrolyzes under acidic or basic conditions | Reverts to formic acid and methanol |
Hydrated esters are reactive intermediates. When exposed to acids or bases, they undergo hydrolysis, decomposing into their original components. This reversible reaction forms the foundation for organic synthesis in laboratories and industrial chemical plants.
Industrial and Environmental Significance
Beyond laboratory exercises, compounds structurally related to hcooch ch2 h2o play crucial roles in chemical manufacturing. Esters and their hydrated derivatives are used as solvents, flavoring agents, and intermediates in polymer production. In the environmental context, they appear in atmospheric aerosols and volatile organic compound (VOC) reactions, influencing air quality and climate chemistry.
Dr. Anita Bose, an environmental chemist at the Indian Institute of Science, notes:
“These small molecules are the invisible workers of the atmosphere. Their breakdown contributes to formaldehyde and carbon monoxide formation—key components in the photochemical smog equation.”
Such statements underscore the balance between utility and caution. While industrially valuable, these compounds demand safe handling and monitoring.
Educational Relevance and Laboratory Practice
hcooch ch2 h2o provides an ideal teaching tool for chemistry students. It allows educators to demonstrate:
- Esterification and hydrolysis mechanisms
- Hydrogen bonding in aqueous systems
- The principle of dynamic equilibrium
- Temperature effects on reaction kinetics
Laboratory demonstrations often involve the preparation of methyl formate, followed by hydration to show changes in boiling points and infrared absorption spectra. Students learn not only reaction pathways but also the real-world significance of small structural variations in molecules.
| Concept | Demonstration Example | Educational Outcome |
|---|---|---|
| Esterification | Formic acid + methanol → methyl formate | Understand condensation reactions |
| Hydration | Adding water to methyl formate | Explore equilibrium and hydrogen bonding |
| Spectroscopy | Observe IR peaks near 1720 cm⁻¹ (C=O stretch) | Identify functional groups |
| Hydrolysis | Use acid catalyst | Link pH to reaction rate |
Research Applications and Future Directions
Modern research explores hydrated esters for their role in biofuel chemistry, green solvents, and carbon capture technologies. Their ability to store and release energy efficiently has inspired studies in formic acid fuel cells, where small molecules serve as hydrogen carriers.
According to Dr. Luis Mendoza, a researcher in sustainable energy at ETH Zurich:
“Hydrated esters like hcooch ch2 h2o embody the kind of chemical efficiency we need for renewable energy systems—lightweight, reactive, and recyclable.”
As the world transitions toward carbon-neutral energy sources, molecules like this become valuable stepping stones in bridging organic chemistry with clean technology.
Expert Perspectives
Dr. Maria Lopez, Harvard University Behavioral Chemist:
“Chemistry education thrives on accessibility. Molecules like HCOOCH₂H₂O show students that complexity lies in simplicity—the same reaction that forms perfume scents also fuels renewable energy debates.”
Prof. David Klein, author of Organic Chemistry Principles:
“Understanding hydration equilibria teaches students chemical intuition—the ability to predict outcomes before running an experiment.”
Dr. Hannah Reed, Educational Psychologist:
“In chemistry pedagogy, contextual learning—connecting molecules to climate, health, or industry—improves retention and critical thinking.”
Key Takeaways
- HCOOCH₂H₂O represents a hydrated ester central to understanding organic reaction dynamics.
- It bridges theoretical chemistry and industrial application—from fuel cells to environmental modeling.
- Hydrogen bonding significantly affects its physical properties and reactivity.
- Educational labs use this compound to teach esterification, equilibrium, and spectroscopy.
- Its study supports sustainable energy research and green chemistry innovation.
- Experts emphasize its importance as a gateway to advanced organic concepts.
- Understanding its chemistry cultivates both academic insight and environmental responsibility.
Conclusion
HCOOCH₂H₂O is more than a cryptic chemical formula—it’s a microcosm of modern science. From classrooms to research labs, it links fundamental theory to global innovation. Its reactions embody the essence of chemistry: transformation, equilibrium, and interconnectedness.
As the planet seeks sustainable answers, understanding molecules like this becomes vital. Each hydrated ester synthesized in a beaker mirrors larger transformations—between carbon and energy, knowledge and progress. In that sense, chemistry remains humanity’s most eloquent dialogue with nature, and hcooch ch2 h2o, though small, speaks volumes about the power of understanding matter at its most intimate scale.
FAQs
1. What is HCOOCH₂H₂O?
It’s a hydrated ester derived from formic acid and methanol, representing a molecular equilibrium between an ester and water.
2. How is it formed?
Through condensation between formic acid and methanol, followed by partial hydration under specific conditions.
3. Why is it important in education?
It’s used to teach esterification, hydrolysis, and equilibrium principles in chemistry labs.
4. Does it occur naturally?
Yes, similar compounds occur transiently in atmospheric chemistry and biological processes.
5. What are its industrial uses?
Analogous esters serve as solvents, fuel intermediates, and agents in biodegradable material synthesis.
References (APA Style)
Bose, A. (2024). Atmospheric Chemistry of Small Esters. Indian Institute of Science Publications.
Hart, E. (2025, September 4). Interview with James Holloway. University of Manchester.
Klein, D. (2023). Organic Chemistry Principles. New York: Pearson.
Lopez, M. (2024). Behavioral Aspects of Science Education. Harvard Education Press.
Mendoza, L. (2025). Renewable Pathways: Molecular Fuel Systems. ETH Zurich Press.
Reed, H. (2024). Chemistry and Cognition: Teaching the Invisible. Oxford University Press.